In terms of commercial electrocatalytic reactions,
there are actually very few. There are
electrowinning reactions, (i.e. electrochemical
reduction of metals salts), mostly notably the
Hall-Heroult process for aluminum, and the
electrosynthesis of halides, but beyond th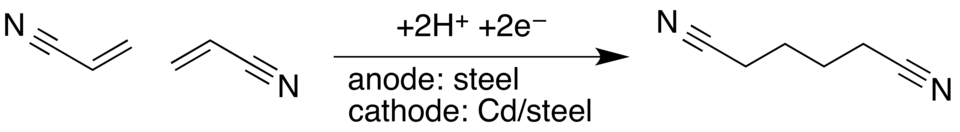 at
there is very little. Though water
electrolysis to hydrogen is often hyped, most
electrocatalytic hydrogen comes as a byproduct of
chlorine evolution. In terms of organic
chemistry, there is really only one notable process
and that is the adiponitriled process as shown on
the right. Thus it is possible for the
economics to work, but we have just not been able to
find other reactions. One of my research
thrusts is to look in this direction. at
there is very little. Though water
electrolysis to hydrogen is often hyped, most
electrocatalytic hydrogen comes as a byproduct of
chlorine evolution. In terms of organic
chemistry, there is really only one notable process
and that is the adiponitriled process as shown on
the right. Thus it is possible for the
economics to work, but we have just not been able to
find other reactions. One of my research
thrusts is to look in this direction. |
|
Valuable products One of the main reasons we have not been able to find economically viable electrosynthesis processes is simply because we have not cared to focus on it. A large amount of electrosynthesis research and development funding has been on fuels and bulk chemicals due to their large market value. However the lack of an emerging H2 economy after decades of effort has shown that another approach is needed. CO2 electrolysis does provide the potential for niche products such as isotopically labelled ethanol and ethylene synthesis (which I am involved in a start-up with), and I believe this can be a starting point for other products as well. While bulk chemicals such as ethanol and ethylene are 2.5 times more valuable than hydrogen on a $/MWh basis, other organics have the potential to be even higher. Aqueous based electrolytes are almost exclusively used in electrochemistry, however organics are not soluble in aquoeous solutions, thus we need to operate in non-aqueous studies. We have had a project recently analyzing CO2 electrolysis is non-aqueous solutions and our biggest finding was that while aqueous reactions allow for catalytic based inner-sphere reactions (i.e. sharing electron density with a catalysts), switching to non-aqueous reactions tend to favor outer-sphere reactions (i.e. non-catalytic), and thus much lower energy efficiencies. Thus one of our major goals is to figure out how to resolve this. Integrating electrochemistry into Nylon synthesis 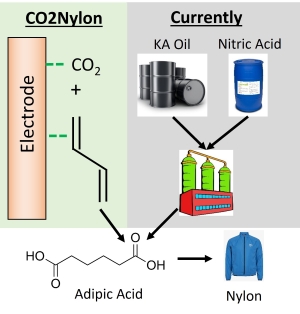 About
6-7M tons of Nylon is produced every year, and this
is derived from reacting hexamethylenediamine and
adipic acid. Adipic acid is a 6-carbon chain
molecule that is typically produced via KA Oil
(fossil fuel derived) and nitric acid
(environmentally unfriendly) . Interestingly,
a few research works have shown that CO2
can be electrochemically reduced onto butadiene to
form adipic acid. While butadiene is normally
produced via fossil fuels, there is a non-negligible
market share that is produced via dehyrogenation of
ethanol, thus providing a sustainable approach to
butadiene. About
6-7M tons of Nylon is produced every year, and this
is derived from reacting hexamethylenediamine and
adipic acid. Adipic acid is a 6-carbon chain
molecule that is typically produced via KA Oil
(fossil fuel derived) and nitric acid
(environmentally unfriendly) . Interestingly,
a few research works have shown that CO2
can be electrochemically reduced onto butadiene to
form adipic acid. While butadiene is normally
produced via fossil fuels, there is a non-negligible
market share that is produced via dehyrogenation of
ethanol, thus providing a sustainable approach to
butadiene.This approach has substantial potential for economic viability, however the CO2 is currently reduced onto the butadiene via a high overpotential outer-sphere radical reaction rather than a more efficient catalytically driven inner-sphere reaction. Thus our goal is to work to achieve a catalytically active coupling of the CO2 and the butadiene. From a more fundamental aspect, this work will analyze how alkene behaves on an electrocatalyst at an applied potential, especially when compared to competing adsorbates such as hydrogen and CO. Given that alkenes is a pretty large class of molecules the overall scientific understanding could be quite broadly applicable. |
CO2
electrolysis coupled with amines
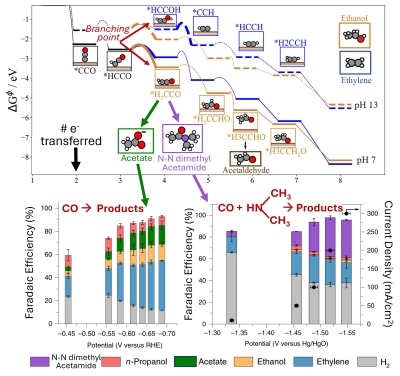 In
2019 a colleague of mine,
Professor Feng Jiao, was trying to analyze the mechanism
of CO2
electrolysis and inserted amines into the electrolyte to
help see if any of the intermediates would react with the
amine to form an amide. Fortunately it did and
this study, along with some of
our studies,
and further
studies by Professor Jiao & DTU computational chemists
all led to a pretty clear indication that CO2
electrolysis proceeds through ethenone, which then would
either desorb and hydrolyze to acetate or get further
reduced on the electrode to ethanol. This also demonstrated
that the step before the ethenone intermediate was the
branching point between ethanol and ethylene, the 2 major
products from Cu based CO2
electrolysis. This concept is shown on the figure to
the right, which is a mesh of
Jouny et al. Nat Chem. 2019. and
Kastlunger et al. ACS Cat 2023.
In
2019 a colleague of mine,
Professor Feng Jiao, was trying to analyze the mechanism
of CO2
electrolysis and inserted amines into the electrolyte to
help see if any of the intermediates would react with the
amine to form an amide. Fortunately it did and
this study, along with some of
our studies,
and further
studies by Professor Jiao & DTU computational chemists
all led to a pretty clear indication that CO2
electrolysis proceeds through ethenone, which then would
either desorb and hydrolyze to acetate or get further
reduced on the electrode to ethanol. This also demonstrated
that the step before the ethenone intermediate was the
branching point between ethanol and ethylene, the 2 major
products from Cu based CO2
electrolysis. This concept is shown on the figure to
the right, which is a mesh of
Jouny et al. Nat Chem. 2019. and
Kastlunger et al. ACS Cat 2023.
However what was forgotten in all this mechanistic understanding was that CO2 electrolysis could take amines and produce amides. Furthermore we repeated some of the experiments and could reproduce their results. Amines is a very broad class of chemicals, and the thought is that we can find an amine that can interact with the ethenone to form a high value product. Thus the goal of this direction is to both understand how the amide is formed and to produce a proudct of value.

